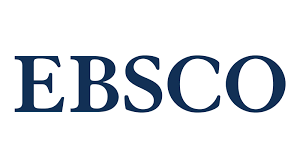Інгібітори фактору росту ендотелію судин при ексудативних захворюваннях сітківки: огляд досягнень останніх років та перспективи подальшого розвитку
DOI:
https://doi.org/10.31288/oftalmolzh202445864Ключові слова:
анти-VEGF препарати, інгібітори фактору росту ендотелію судин, біофармацевтичні препарати, біосиміляри, фармакологія, неоваскулярна вікова макулярна дегенерація, ексудативні захворювання сітківки, сітківкаАнотація
За 20 років застосування інгібіторів фактору росту ендотелію судин (анти-VEGF препаратів) в офтальмології вдалося значно зменшити кількість випадків сліпоти внаслідок ексудативних захворювань сітківки. Однак незважаючи на досягнення останніх років, поточні результати анти-VEGFтерапії не завжди є оптимальними. Метою даного огляду наукових публікацій є висвітлення досягнень у розробці інгібіторів фактору росту ендотелію судин та визначення потенційних шляхів покращення результатів анти-VEGF терапії й подолання поточних проблем у лікуванні ексудативних захворювань сітківки.
Посилання
GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021 Feb;9(2):e144-e160. doi: 10.1016/S2214-109X(20)30489-7. Epub 2020 Dec 1. Erratum in: Lancet Glob Health. 2021 Apr;9(4):e408.
Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-Related Macular Degeneration: A Review. JAMA. 2024 Jan 9;331(2):147-157. doi: 10.1001/jama.2023.26074. PMID: 38193957. https://doi.org/10.1001/jama.2023.26074
Nozaki M, Ando R, Kimura T, Kato F, Yasukawa T. The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review. Medicina (Kaunas). 2023 Jul 17;59(7):1319. https://doi.org/10.3390/medicina59071319
Wang B, Zhang X, Chen H, Koh A, Zhao C, Chen Y. A Review of Intraocular Biomolecules in Retinal Vein Occlusion: Toward Potential Biomarkers for Companion Diagnostics. Front Pharmacol. 2022 Apr 26;13:859951. https://doi.org/10.3389/fphar.2022.859951
Chen Y, Han X, Gordon I, Safi S, Lingham G, Evans J, Li J, He M, Keel S. A systematic review of clinical practice guidelines for myopic macular degeneration. J Glob Health. 2022 Mar 26;12:04026. https://doi.org/10.7189/jogh.12.04026
Lanzetta P. Anti-VEGF therapies for age-related macular degeneration: a powerful tactical gear or a blunt weapon? The choice is ours. Graefes Arch Clin Exp Ophthalmol. 2021 Dec;259(12):3561-3567. Epub 2021 Oct 20. https://doi.org/10.1007/s00417-021-05451-2
Khachigian LM, Liew G, Teo KYC, Wong TY, Mitchell P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J Transl Med. 2023 Feb 21;21(1):133. https://doi.org/10.1186/s12967-023-03937-7
Instruction for medical use of the medicinal product EYLEA® (UA/12600/01/01), available in the State Register of Medicinal Products of Ukraine (http://drlz.com.ua) as of March 2014.
Instruction for medical use of the medicinal product VSIQQ (UA/18833/01/01), available in the State Register of Medicinal Products of Ukraine (http://drlz.com.ua) as of March 2014.
Instruction for medical use of the medicinal product LUCENTIS (UA/9924/01/01), available in the State Register of Medicinal Products of Ukraine (http://drlz.com.ua) as of March 2014.
Instruction for medical use of the medicinal product VABISMO (UA/20151/01/01), available in the State Register of Medicinal Products of Ukraine (http://drlz.com.ua) as of March 2014.
Regeneron Pharmaceuticals Inc. EYLEA - prescribing information; Revised: December 2023.
Bayer AG. EYLEA - summary of product characteristics; Last updated: 20/03/2024.
Bayer Yakuhin, Ltd.; Santen Pharmaceutical Co., Ltd. Press release March 25, 2020. Available from: https://ssl4.eir-parts.net/doc/4536/tdnet/1809806/00.pdf
VABYSMO™ (faricimab-svoa) Prescribing Information. San Francisco, USA: Genentech, Inc. Revised: 10/2023
Singh RP, Avery RL, Barakat MR, Kim JE, Kiss S. Evidence-Based Use of Bevacizumab in the Management of Neovascular Age-Related Macular Degeneration. Ophthalmic Surg Lasers Imaging Retina. 2024 Mar;55(3):156-162. Epub 2024 Mar 1. https://doi.org/10.3928/23258160-20240108-01
Zur D, Hod K, Trivizki O, Rabinovitch D, Schwartz S, Shulman S. Anti-Vascular Endothelial Growth Factor Treatment in Diabetic Macular Edema-Results from a Large Single Center Cohort with Bevacizumab As First-Line Therapy. Retina. 2024 Mar 12. Epub ahead of print. https://doi.org/10.1097/IAE.0000000000004096
Outlook Therapeutics® Receives European Commission Marketing Authorization for LYTENAVA™ (bevacizumab gamma) for the Treatment of Wet AMD. Available from: https://ir.outlooktherapeutics.com/news-releases/news-release-details/outlook-therapeuticsr-receives-european-commission-marketing
Huang YT, Tien PT, Chen PY, Yang CL, Chen SN. Comparative efficacy of brolucizumab, half-dose photodynamic therapy, and aflibercept in managing chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2024 Jan 15. doi: 10.1007/s00417-024-06373-5. Epub ahead of print. https://doi.org/10.1007/s00417-024-06373-5
Romdhane K, Zola M, Matet A, Daruich A, Elalouf M, Behar-Cohen F, Mantel I. Predictors of treatment response to intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy for choroidal neovascularisation secondary to chronic central serous chorioretinopathy. Br J Ophthalmol. 2020 Jul;104(7):910-916. Epub 2019 Oct 15. https://doi.org/10.1136/bjophthalmol-2019-314625
Laich Y, Georgiou M, Fujinami K, Daich Varela M, Fujinami-Yokokawa Y, Hashem SA, Cabral de Guimaraes TA, Mahroo OA, Webster AR, Michaelides M. Best Vitelliform Macular Dystrophy Natural History Study Report 1: Clinical Features and Genetic Findings. Ophthalmology. 2024 Jan 24:S0161-6420(24)00086-1. Epub ahead of print. https://doi.org/10.1016/j.ophtha.2024.01.027
Adiyeke SK, Ture G. Choroidal Neovascularization Associated with Best Vitelliform Macular Dystrophy. Beyoglu Eye J. 2022 May 27;7(2):103-108. https://doi.org/10.14744/bej.2022.54376
Tsokolas G, Tossounis C, Tyradellis S, Motta L, Panos GD, Empeslidis T. Angioid Streaks Remain a Challenge in Diagnosis, Management, and Treatment. Vision (Basel). 2024 Mar 5;8(1):10. https://doi.org/10.3390/vision8010010
Gliem M, Birtel J, Herrmann P, Fimmers R, Berger M, Coch C, Wingen A, Holz FG, Charbel Issa P. Aflibercept for choroidal neovascularizations secondary to pseudoxanthoma elasticum: a prospective study. Graefes Arch Clin Exp Ophthalmol. 2020 Feb;258(2):311-318. Epub 2019 Dec 20. https://doi.org/10.1007/s00417-019-04551-4
Korol AR, Zborovska O, Kustryn T, Dorokhova O, Pasyechnikova N. Intravitreal aflibercept for choroidal neovascularization associated with chorioretinitis: a pilot study. Clin Ophthalmol. 2017 Jul 20;11:1315-1320. Erratum in: Clin Ophthalmol. 2017 Aug 28;11:1567. https://doi.org/10.2147/OPTH.S132923
Orski M, Gawęcki M. Current Management Options in Irvine-Gass Syndrome: A Systemized Review. J Clin Med. 2021 Sep 25;10(19):4375. https://doi.org/10.3390/jcm10194375
Akay F, Işık MU, Akmaz B, Güven YZ. Comparison of intravitreal anti-vascular endothelial growth factor agents and treatment results in Irvine-Gass syndrome. Int J Ophthalmol. 2020 Oct 18;13(10):1586-1591. https://doi.org/10.18240/ijo.2020.10.12
Bottini AR, Blackorby BL, Michaels M, Burkett K, Dang S, Blinder KJ, Shah GK. Long-Term Outcomes in Macular Telangiectasia Type 2 With Subretinal Neovascularization. J Vitreoretin Dis. 2020 Jun 17;4(5):386-392. https://doi.org/10.1177/2474126420927149
Sen S, Rajan RP, Damodaran S, Arumugam KK, Kannan NB, Ramasamy K. Real-world outcomes of intravitreal anti-vascular endothelial growth factor monotherapy in proliferative type 2 macular telangiectasia. Graefes Arch Clin Exp Ophthalmol. 2021 May;259(5):1135-1143. Epub 2020 Nov 17. https://doi.org/10.1007/s00417-020-05007-w
Li L, Li S, Liu J, Deng G, Ma J, Lu H. Long-term efficacy and complications of intravitreal anti-vascular endothelial growth factor agents combined with ablative therapies in juvenile Coats disease: a five year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2024 Jan;262(1):305-312. Epub 2023 Jul 8. https://doi.org/10.1007/s00417-023-06162-6
Alsaggaf K, Jalloun M, Alkhotani W, Albeedh M. Three-Year Results of Management of Adult-Onset Coats' Disease by Possibly Targeting Placental Growth Factor. Cureus. 2020 Sep 25;12(9):e10652. https://doi.org/10.7759/cureus.10652
Ponomarchuk V, Velichko L, Korol A, Umanets N. [Concentration of Vascular Endothelial Growth Factor in the Vitreous and Features of Vitrectomy in Patients with Proliferative Diabetic Retinopathy after Various Doses of Intravitreal Aflibercept]. Oftalmologiia. Vostochnaia Evropa. 2022;12(1):98-107. Russian. https://doi.org/10.34883/PI.2022.12.1.026
Marino M, Jamal Z, Zito PM. Pharmacodynamics. [Updated 2023 Jan 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507791/
Veritti D, Sarao V, Gorni G, Lanzetta P. Anti-VEGF Drugs Dynamics: Relevance for Clinical Practice. Pharmaceutics. 2022 Jan 23;14(2):265. https://doi.org/10.3390/pharmaceutics14020265
Arrigo A, Bandello F. Molecular Features of Classic Retinal Drugs, Retinal Therapeutic Targets and Emerging Treatments. Pharmaceutics. 2021 Jul 20;13(7):1102. https://doi.org/10.3390/pharmaceutics13071102
García-Quintanilla L, Luaces-Rodríguez A, Gil-Martínez M, Mondelo-García C, Maroñas O, Mangas-Sanjuan V, González-Barcia M, Zarra-Ferro I, Aguiar P, Otero-Espinar FJ, Fernández-Ferreiro A. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics. 2019 Jul 31;11(8):365. https://doi.org/10.3390/pharmaceutics11080365
Lamminsalo M, Urtti A, Ranta VP. Quantitative pharmacokinetic analyses of anterior and posterior elimination routes of intravitreal anti-VEGF macromolecules using published human and rabbit data. Exp Eye Res. 2022 Sep;222:109162. doi: 10.1016/j.exer.2022.109162. Epub 2022 Jun 26. https://doi.org/10.1016/j.exer.2022.109162
Schubert W, Terjung C, Rafique A, Romano C, Ellinger P, Rittenhouse KD. Evaluation of Molecular Properties versus In Vivo Performance of Aflibercept, Brolucizumab, and Ranibizumab in a Retinal Vascular Hyperpermeability Model. Transl Vis Sci Technol. 2022 Oct 3;11(10):36. https://doi.org/10.1167/tvst.11.10.36
Ross AH, Downey L, Devonport H, Gale RP, Kotagiri A, Mahmood S, Mehta H, Narendran N, Patel PJ, Parmar N, Jain N. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye (Lond). 2020 Oct;34(10):1825-1834. Epub 2020 Jan 3.. https://doi.org/10.1038/s41433-019-0747-x
Eylea HD (aflibercept) injection 8 mg approved by FDA for treatment of wet age-related macular degeneration (WAMD), diabetic macular edema (DME) and diabetic retinopathy (DR). Available from: https://investor.regeneron.com/news-releases/news-release-details/eylea-hd-aflibercept-injection-8-mg-approved-fda-treatment-wet
New Eylea™ 8 mg approved in EU. Available from: https://www.bayer.com/media/en-us/new-eylea-8-mg-approved-in-eu/
Batsos G, Christodoulou E, Christou EE, Galanis P, Katsanos A, Limberis L, Stefaniotou M. Vitreous inflammatory and angiogenic factors on patients with proliferative diabetic retinopathy or diabetic macular edema: the role of Lipocalin2. BMC Ophthalmol. 2022 Dec 19;22(1):496. https://doi.org/10.1186/s12886-022-02733-z
Gong QY, Hu GY, Yu SQ, Qian TW, Xu X. Comprehensive assessment of growth factors, inflammatory mediators, and cytokines in vitreous from patients with proliferative diabetic retinopathy. Int J Ophthalmol. 2022 Nov 18;15(11):1736-1742. https://doi.org/10.18240/ijo.2022.11.02
Lange C, Tetzner R, Strunz T, Rittenhouse KD. Aflibercept Suppression of Angiopoietin-2 in a Rabbit Retinal Vascular Hyperpermeability Model. Transl Vis Sci Technol. 2023 May 1;12(5):17. https://doi.org/10.1167/tvst.12.5.17
Guo J, Liu ZH, Pan M, An GQ, Du LP, Zhou PY, Jin XM. [The effect of anti-VEGF therapy on the expression levels of TGF-β and related microRNAs in the vitreous of patients with proliferative diabetic retinopathy]. Zhonghua Yan Ke Za Zhi. 2021 Dec 11;57(12):922-929. Chinese. doi: 10.3760/cma.j.cn112142-20210317-00133.
Martínez-Vacas A, Di Pierdomenico J, Gómez-Ramirez AM, Vidal-Sanz M, Villegas-Pérez MP, García-Ayuso D. Dose-Related Side Effects of Intravitreal Injections of Humanized Anti-Vascular Endothelial Growth Factor in Rats: Glial Cell Reactivity and Retinal Ganglion Cell Loss. Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):10. https://doi.org/10.1167/iovs.65.4.10
Juncal VR, Francisconi CLM, Altomare F, Chow DR, Giavedoni LR, Muni RH, Berger AR, Wong DT. Same-Day Bilateral Intravitreal Anti-Vascular Endothelial Growth Factor Injections: Experience of a Large Canadian Retina Center. Ophthalmologica. 2019;242(1):1-7. Epub 2019 Mar 29. https://doi.org/10.1159/000499115
Bjerager J, Hajari J, Klefter ON, Subhi Y, Schneider M. Systemic adverse events and all-cause mortality following same-session bilateral intravitreal anti-VEGF injections: a systematic review. Graefes Arch Clin Exp Ophthalmol. 2024 Jan 9. doi: 10.1007/s00417-023-06368-8. Epub ahead of print. https://doi.org/10.1007/s00417-023-06368-8
EMA. Biosimilars in the EU: information guide for healthcare professionals. Available from: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf
EMA. Byooviz (ranibizumab). Available at: https://www.ema.europa.eu/en/documents/overview/byooviz-epar-medicine-overview_en.pdf.
Formycon AG. Formycon announces EU-approval of FYB201/Ranivisio® a biosimilar to Lucentis®. Available from: https://www.formycon.com/en/blog/press-release/formycon-announces-eu-approval-of-fyb201-ranivisio1-a-biosimilar-to-lucentis2/.
Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Biotherapeutics and immunogenicity: ophthalmic perspective. Eye (Lond). 2019 Sep;33(9):1359-1361. Epub 2019 Apr 9. https://doi.org/10.1038/s41433-019-0434-y
Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Ophthalmic biosimilars and biologics-role of endotoxins. Eye (Lond). 2020 Apr;34(4):614-615. Epub 2019 Oct 16. https://doi.org/10.1038/s41433-019-0636-3
Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, Eldem B, Monés J, Richard G, Bandello F; European Society of Retina Specialists. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014 Sep;98(9):1144-67. doi: 10.1136/bjophthalmol-2014-305702. https://doi.org/10.1136/bjophthalmol-2014-305702
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, Jonas J, Larsen M, Tadayoni R, Loewenstein A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185-222. https://doi.org/10.1159/000458539
Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, Midena E, Sivaprasad S, Tadayoni R, Wolf S, Loewenstein A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2019;242(3):123-162. Epub 2019 Aug 14. https://doi.org/10.1159/000502041
Grzybowski A, Told R, Sacu S, Bandello F, Moisseiev E, Loewenstein A, Schmidt-Erfurth U; Euretina Board. 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica. 2018;239(4):181-193. Epub 2018 Feb 1. https://doi.org/10.1159/000486145
Iyer PG, Albini TA. Drug-related adverse effects of antivascular endothelial growth factor agents. Curr Opin Ophthalmol. 2021 May 1;32(3):191-197. https://doi.org/10.1097/ICU.0000000000000757
Sharma A, Kumar N, Parachuri N, Singh S, Bandello F, Kuppermann BD, Loewenstein A. Brolucizumab-related retinal vasculitis: emerging disconnect between clinical trials and real world. Eye (Lond). 2021 May;35(5):1292-1294. Epub 2020 Oct 20. https://doi.org/10.1038/s41433-020-01227-w
DHCP Important Drug Warning: VABYSMO® (faricimab-svoa), New Warnings and Precautions: Retinal Vasculitis and/or Retinal Vascular Occlusion. Available from: https://www.gene.com/download/pdf/Vabysmo_DHCP_Important_Drug_Warning_2023-11-03.pdf.
Monés J, Srivastava SK, Jaffe GJ, Tadayoni R, Albini TA, Kaiser PK, Holz FG, Korobelnik JF, Kim IK, Pruente C, Murray TG, Heier JS. Risk of Inflammation, Retinal Vasculitis, and Retinal Occlusion-Related Events with Brolucizumab: Post Hoc Review of HAWK and HARRIER. Ophthalmology. 2021 Jul;128(7):1050-1059. Epub 2020 Nov 15. https://doi.org/10.1016/j.ophtha.2020.11.011
Stern HD, Hussain RM. KSI-301: an investigational anti-VEGF biopolymer conjugate for retinal diseases. Expert Opin Investig Drugs. 2022 May;31(5):443-449. Epub 2022 Mar 16. https://doi.org/10.1080/13543784.2022.2052042
KSI-501: A Novel Bispecific Antibody Biopolymer Conjugate Targeting IL-6 and VEGF. Clinical Trials at the Summit 2023. Available from: https://ir.kodiak.com/static-files/113a6a55-e305-4d89-abf7-dbbfd55ec1ac
Jackson TL, Slakter J, Buyse M, Wang K, Dugel PU, Wykoff CC, Boyer DS, Gerometta M, Baldwin ME, Price CF; Opthea Study Group Investigators. A Randomized Controlled Trial of OPT-302, a VEGF-C/D Inhibitor for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2023 Jun;130(6):588-597. Epub 2023 Feb 6. https://doi.org/10.1016/j.ophtha.2023.02.001
OPT-302 With Aflibercept in Neovascular Age-related Macular Degeneration (nAMD) (COAST). Available from: https://clinicaltrials.gov/study/NCT04757636
OPT-302 With Ranibizumab in Neovascular Age-related Macular Degeneration (nAMD) (ShORe). Available from: https://clinicaltrials.gov/study/NCT04757610
Campochiaro PA, Avery R, Brown DM, Heier JS, Ho AC, Huddleston SM, Jaffe GJ, Khanani AM, Pakola S, Pieramici DJ, Wykoff CC, Van Everen S. Gene therapy for neovascular age-related macular degeneration by subretinal delivery of RGX-314: a phase 1/2a dose-escalation study. Lancet. 2024 Apr 20;403(10436):1563-1573. Epub 2024 Mar 27. https://doi.org/10.1016/S0140-6736(24)00310-6
Khanani AM, Boyer DS, Wykoff CC, Regillo CD, Busbee BG, Pieramici D, Danzig CJ, Joondeph BC, Major JC Jr, Turpcu A, Kiss S. Safety and efficacy of ixoberogene soroparvovec in neovascular age-related macular degeneration in the United States (OPTIC): a prospective, two-year, multicentre phase 1 study. EClinicalMedicine. 2023 Dec 22;67:102394. https://doi.org/10.1016/j.eclinm.2023.102394
Lima E Silva R, Mirando AC, Tzeng SY, Green JJ, Popel AS, Pandey NB, Campochiaro PA. Anti-angiogenic collagen IV-derived peptide target engagement with αvβ3 and α5β1 in ocular neovascularization models. iScience. 2023 Jan 30;26(2):106078. https://doi.org/10.1016/j.isci.2023.106078
Yang S, Li T, Jia H, Gao M, Li Y, Wan X, Huang Z, Li M, Zhai Y, Li X, Yang X, Wang T, Liang J, Gu Q, Luo X, Qian L, Lu S, Liu J, Song Y, Wang F, Sun X, Yu D. Targeting C3b/C4b and VEGF with a bispecific fusion protein optimized for neovascular age-related macular degeneration therapy. Sci Transl Med. 2022 Jun;14(647):eabj2177. Epub 2022 Jun 1. https://doi.org/10.1126/scitranslmed.abj2177
PanOptica Anti-VEGF Eye Drop Shows Promise in Treatment ofNeovascular (Wet) AMD. Available from: https://www.panopticapharma.com/wp-content/uploads/2019/10/PanOptica-PAN-90806-Data-Summary-Release-Clean-Final-Oct-09-2019.pdf
Graybug Vision Reports Preliminary Topline Results from Phase 2b ALTISSIMO Trial. Available from: https://graybug.gcs-web.com/news-releases/news-release-details/graybug-vision-reports-preliminary-topline-results-phase-2b
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2024 Зайченко Г. В., Розумний В. П.

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Ця робота ліцензується відповідно до ліцензії Creative Commons Attribution 4.0 International (CC BY). Ця ліцензія дозволяє повторно використовувати, поширювати, переробляти, адаптувати та будувати на основі матеріалу на будь-якому носії або в будь-якому форматі за умови обов'язкового посилання на авторів робіт і первинну публікацію у цьому журналі. Ліцензія дозволяє комерційне використання.
ПОЛОЖЕННЯ ПРО АВТОРСЬКІ ПРАВА
Автори, які подають матеріали до цього журналу, погоджуються з наступними положеннями:
- Автори отримують право на авторство своєї роботи одразу після її публікації та назавжди зберігають це право за собою без жодних обмежень.
- Дата початку дії авторського права на статтю відповідає даті публікації випуску, до якого вона включена.
ПОЛІТИКА ДЕПОНУВАННЯ
- Редакція журналу заохочує розміщення авторами рукопису статті в мережі Інтернет (наприклад, у сховищах установ або на особистих веб-сайтах), оскільки це сприяє виникненню продуктивної наукової дискусії та позитивно позначається на оперативності і динаміці цитування.
- Автори мають право укладати самостійні додаткові угоди щодо неексклюзивного розповсюдження статті у тому вигляді, в якому вона була опублікована цим журналом за умови збереження посилання на первинну публікацію у цьому журналі.
- Дозволяється самоархівування постпринтів (версій рукописів, схвалених до друку в процесі рецензування) під час їх редакційного опрацювання або опублікованих видавцем PDF-версій.
- Самоархівування препринтів (версій рукописів до рецензування) не дозволяється.