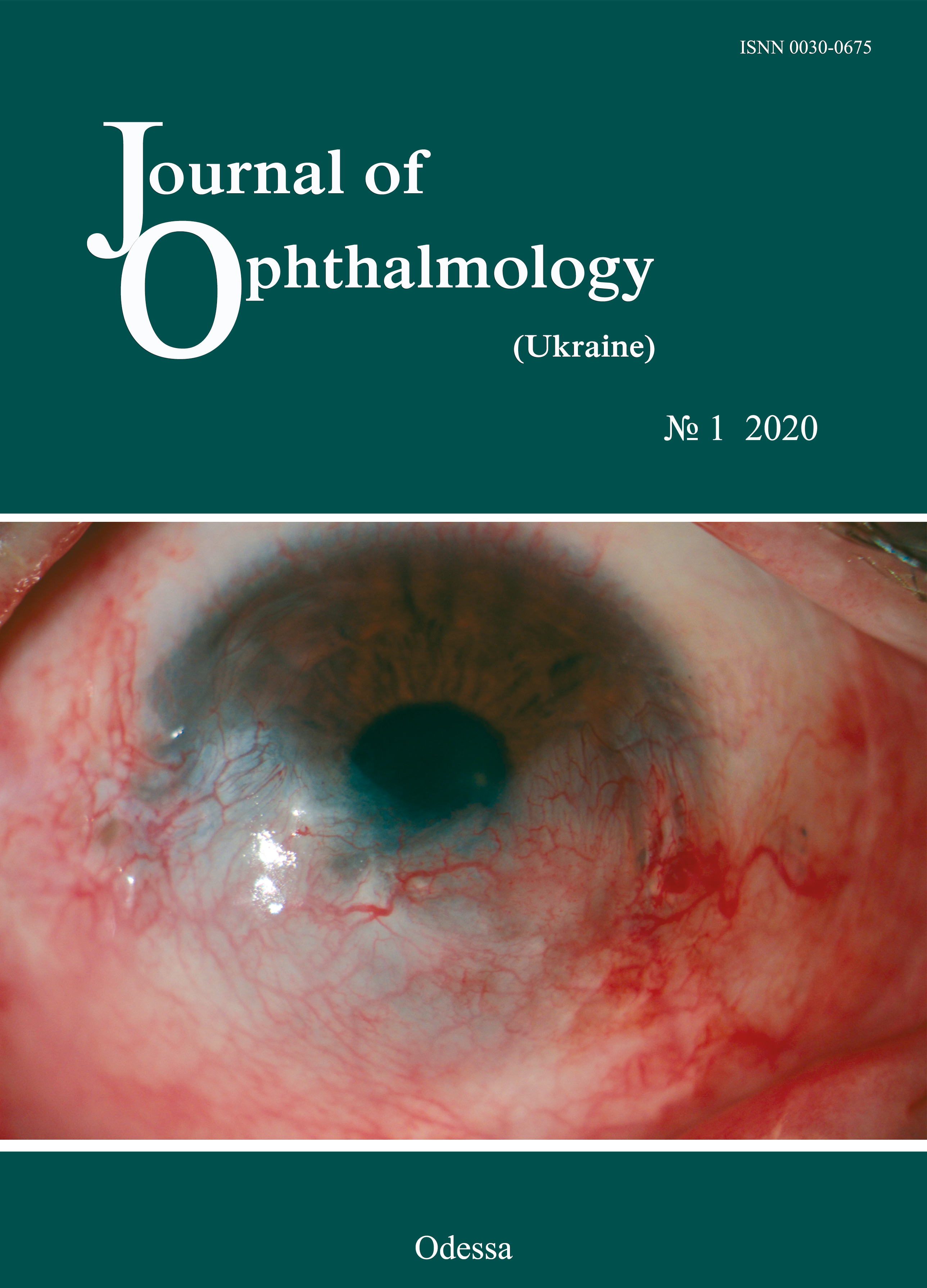
A case of vascularized corneal opacity treated with aflibercept
DOI:
https://doi.org/10.31288/oftalmolzh202015456Keywords:
corneal neovascularization, fluorescein angiography, angiogenesis inhibitor, afliberceptAbstract
Background: Corneal neovascularization (CNV) is a serious condition that can lead to a profound decline in vision. Current research on treating CNV with angiogenesis inhibitors is underway in numerous centers, and a number of studies have demonstrated the efficacy of this approach.
Purpose: To present a case of corneal neovascularization treated with aflibercept.
Material and Methods: An ocular examination included visual acuity assessment, biomicroscopy, anterior eye photography and fluorescein angiography (FA). In addition, an area of CNV was assessed before and 6 months after a single subconjunctival injection of 4 mg (0.1ml) aflibercept.
Results: An area of CNV decreased from 38,081 pixels before injection to 20,782 at 6 months, with a reduction in 17,299 pixels.
Conclusion: In the case reported here, a single subconjunctival injection of 4 mg (0.1ml) aflibercept was found to be effective for reducing corneal neovascularization at 6 weeks.
References
1.Puchkovskaia NA, Iakimenko SA, Nepomiashchaia VM. [Eye burns]. Moscow: Meditsina; 2001. Russian.
2.Chang JH, Garg NK, Lunde E, et al. Corneal Neovascularization: An Anti-VEGF Therapy Review. Surv Ophthalmol. 2012;57(5):415-29.https://doi.org/10.1016/j.survophthal.2012.01.007
3.Hashemian MN, Zare MA, Rahimi F, et al. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011 Feb;30(2):215-8. https://doi.org/10.1097/ICO.0b013e3181e291a6
4.Ulyanov VA, Makarova MB, Molchaniuk NI, et al. [Effect of colloidal silver nanoparticle solution instillation on the ultrastructure of the anterior corneal epithelium and stroma]. J Ophthalmol (Ukraine). 2017; 3:63-9. Russian.
5.Chang JH, Gabison EE, Kato T, et al. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242-249.https://doi.org/10.1097/00055735-200108000-00002
6.Garkava NA, Fedirko PA, Babenko TF, et al. Radiation induced violations of blood circulation in the ciliary body and changes of the anterior chamber angle in the pathogenesis of glaucoma in clean-up workers of the chornobyl NPP accident and residents of contaminated areas. Probl Radiac Med Radiobiol. 2017 Dec;22:332-338.https://doi.org/10.33145/2304-8336-2017-22-332-338
7.Doctor PP, Bhat PV, Foster CS. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008 Oct;27(9):992-5. https://doi.org/10.1097/ICO.0b013e31817786ad
8.Zaki AA, Farid SF. Subconjunctival bevacizumab for corneal neovascularization. Acta Ophthalmol. Acta Ophthalmol. 2010;88(8):868-71.
https://doi.org/10.1111/j.1755-3768.2009.01585.x
9.Avisar I, Weinberger D, Kremer I. Effect of subconjunctival and intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res. 2010 Feb;35(2):108-15. https://doi.org/10.3109/02713680903429007
10.Bock F, Konig Y, Kruse F, et al. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008 Feb;246(2):281-4. https://doi.org/10.1007/s00417-007-0684-4
11.Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009 Apr;127(4):381-9. https://doi.org/10.1001/archophthalmol.2009.18
12.Sarah B, Ibtissam H, Baali Mohammed et al. Intrastromal Injection of Bevacizumab in the Management of Corneal Neovascularization: About 25 Eyes. J Ophthalmol. 2016;2016:6084270.https://doi.org/10.1155/2016/6084270
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 А. С. Чолак, И. О. Насинник, П. А. Костенко, С. А. Якименко, А. Р. Король

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) that allows users to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author as long as they cite the source.
COPYRIGHT NOTICE
Authors who publish in this journal agree to the following terms:
- Authors hold copyright immediately after publication of their works and retain publishing rights without any restrictions.
- The copyright commencement date complies the publication date of the issue, where the article is included in.
DEPOSIT POLICY
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) during the editorial process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work with an acknowledgement of its initial publication in this journal.
- Post-print (post-refereeing manuscript version) and publisher's PDF-version self-archiving is allowed.
- Archiving the pre-print (pre-refereeing manuscript version) not allowed.