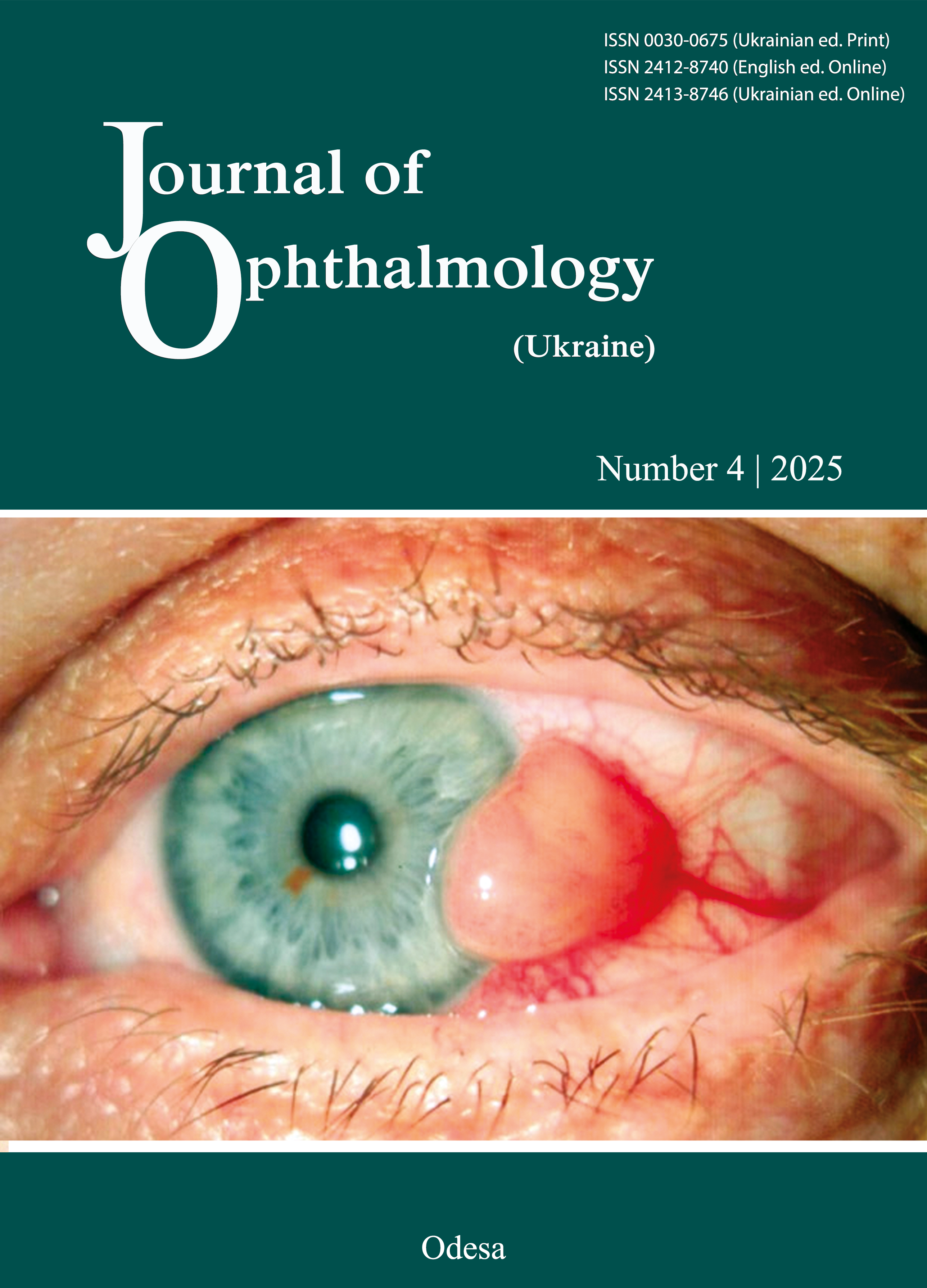
Clinical characteristics of medium and large T1-T4 choroidal melanomas in Ukraine
DOI:
https://doi.org/10.31288/oftalmolzh202543540Keywords:
ophthalmic oncology,, choroidal melanoma,, tumor stage, retina, choroid, transpupillary therapy, ionizing radiationAbstract
Purpose. To evaluate the clinical characteristics of medium to large Т1-Т4 choroidal melanomas (CM) in patients treated at SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”.
Material and Methods. We retrospectively reviewed the medical records of 283 patients treated for medium to large CM at SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine” in 2007-2024. The study sample consisted of 125 men (44.2%) and 158 women (55.8%), with a mean age (standard deviation (SD)) of 54.2 (12.4) years. Patients underwent standard ophthalmological examination. Additionally, they received ocular ultrasonography using a Cinescan ultrasound system, OCT examination using a Stratus OCT 300 system and fluorescein angiography. No metastasis was detected in any patient at presentation and the start of treatment.
Results. The case distribution included stage T1 in 15 patients (5.3%), stage T2 in 132 patients (46.64%), stage T3 in 115 patients (40.64%), and stage T4 in 21 patients (7.42%). Paramacular and peripheral locations of CM were more common in the total sample (88.4%) (p <0.05), and stages T2 (59 [44.7%] and 53 [40.15%], respectively) and T3 (60 [52.17%] and 50 [43.48%], respectively), and were the only two locations seen in stage T4 (10 [47.62%] and 11 [52.38%], respectively). Parafoveal and juxtapapillary tumor locations were seen in stages T1 to T3 (10 [3.53%] and 23 [8.13%], respectively, of the 283 patients), and were more common in stage T2 (6 [60.0%] and 14 [60.87%], respectively). Heterogeneously pigmented CM were most common (132 [46.64%]), followed by mildly pigmented (104 [36.75%]), pigmented (41 [14.49%]) and amelanotic (6 [2.12%]). There were no amelanotic cases in stages T1 and T4. Mildly pigmented CM were present among tumors of any stage, and were most common in stage T2 (60 [21.2%]). Heterogeneously pigmented and pigmented CM were present among tumors of any stage, and were more common in stages T3 (62 [53.91%]) and T2 (54 [40.91%]). T4 tumors were predominantly heterogeneously pigmented and pigmented (13 [61.9%] and 5 [23.81%], respectively) (p < 0.05). Among 283 cases of CM, 147 (147 (51.9%) were dome-shaped, 73 (25.8%) were mushroom-shaped, 54 (19.1%) were finger-shaped and 9 (3.2%) were multilobulated. All patients had diffuse tumors without distinct borders, with a secondary retinal detachment above and around the tumor.
Conclusion. We examined the clinical signs depending on the stage of CM. These signs have a prognostic value for the efficacy of treatment, which will be reported subsequently.
References
Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998 Oct 15;83(8):1664-78.https://doi.org/10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G
Gragoudas ES, Egan KM. Uveal melanoma: a rare malignancy. Ophthalmology. 2000 Aug;107(8):1441-2. https://doi.org/10.1016/S0161-6420(00)00288-8
Yonekawa Y, Kim IK. Epidemiology and management of uveal melanoma. Hematol Oncol Clin North Am. 2012 Dec;26(6):1169-84. https://doi.org/10.1016/j.hoc.2012.08.004
Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012 Aug;38(5):549-53. https://doi.org/10.1016/j.ctrv.2012.01.002
Damato B. Treatment of primary intraocular melanoma. Expert Rev Anticancer Ther. 2006 Apr;6(4):493-506. https://doi.org/10.1586/14737140.6.4.493
Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. EUROCARE Working Group. Incidence of uveal melanoma in Europe. Ophthalmology. 2007 Dec;114(12):2309-15. https://doi.org/10.1016/j.ophtha.2007.01.032
Koomen ER, de Vries E, van Kempen LC, van Akkooi AC, Guchelaar HJ, Louwman MW, et al. Epidemiology of extracutaneous melanoma in the Netherlands. Cancer Epidemiol Biomarkers Prev. 2010 Jun;19(6):1453-9. https://doi.org/10.1158/1055-9965.EPI-09-1267
Isager P, Østerlind A, Engholm G, Heegaard S, Lindegaard J, Overgaard J, et al. Uveal and conjunctival malignant melanoma in Denmark, 1943-97: incidence and validation study. Ophthalmic Epidemiol. 2005 Aug;12(4):223-32.https://doi.org/10.1080/09286580591000836
Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011 Sep;118(9):1881-5. https://doi.org/10.1016/j.ophtha.2011.01.040
Saornil MA, Ordonez JL, Almaraz A, et al. Epidemiologic profile of uveal melanoma patients in Spain. 10th International congress of ocular oncology: Final programme and abstract book. Amsterdam, the Netherlands. June 17-20; 2001.
Frenkel S, Hendler K, Pe'er J. Uveal melanoma in Israel in the last two decades: characterization, treatment and prognosis. Isr Med Assoc J. 2009 May;11(5):280-5.
Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 2005 Oct;140(4):612-7. https://doi.org/10.1016/j.ajo.2005.05.034
Weis E, Shah CP, Lajous M, Shields JA, Shields CL. The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol. 2006 Jan;124(1):54-60. https://doi.org/10.1001/archopht.124.1.54
Diener-West M, Reynolds SM, Agugliaro DJ, et al; Collaborative Ocular Melanoma Study Group. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005 Dec;123(12):1639-43. https://doi.org/10.1001/archopht.123.12.1639
Aronow ME, Tophan AR, Singh AD. Uveal melanomes: 5-yearupdated information on incidence, treatment and survival (SEER 1973-2013). Ocul Oncol Pathol; 2018(4):145-151. https://doi.org/10.1159/000480640
Anina IeI, Levtiukh VI. [Restoring vision with surgery and medications. Proceedings of the 12th Symposium in Ophthalmology]. June 29 - July 1, 2001, Chernivtsi, Ukraine. Chernivtsi; 2001. Ukrainian.
Singh AD, Shields CL, Shields JA. Prognostic factors in uveal melanoma. Melanoma Res. 2001 Jun;11(3):255-63. https://doi.org/10.1097/00008390-200106000-00007
Ewens KG, Kanetsky PA, Richards-Yutz J, Al-Dahmash S, De Luca MC, Bianciotto CG, et al. Genomic profile of 320 uveal melanoma cases: chromosome 8p-loss and metastatic outcome. Invest Ophthalmol Vis Sci. 2013 Aug 23;54(8):5721-9.https://doi.org/10.1167/iovs.13-12195
Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res. 2010 Dec 15;16(24):6083-92. https://doi.org/10.1158/1078-0432.CCR-10-2076
Damato B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye (Lond). 2012 Sep;26(9):1157-72. https://doi.org/10.1038/eye.2012.126
American Joint Committee on Cancer - AJCC Cancer Staging Manual, Eighth Edition [Internet]. Last updated 05 June 2018. Available from: http://www.cancerstaging.org.
Buhl A, Zofel P. [SPSS Version 10-Introduction to modern data analysis]. Addison-Wesley: München, Germany; 2000. German.
Tsukanova IV. [Efficacy of modified transpupillary thermotherapy in the treatment of T1N0M0 choroidal melanoma. Thesis for the degree of Cand Sc (Med)]. Odesa: SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; 2021. Ukrainian.
Shields CL, Shields JA, Perez N, Singh AD, Cater J. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002 Feb;109(2):225-34. https://doi.org/10.1016/S0161-6420(01)00902-2
Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr Opin Ophthalmol. 2002 Jun;13(3):135-41.https://doi.org/10.1097/00055735-200206000-00001
Diener-West M, Hawkins BS, Markowitz JA, Schachat AP. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol. 1992 Feb;110(2):245-50.https://doi.org/10.1001/archopht.1992.01080140101036
Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997 Jul;115(7):886-93.https://doi.org/10.1001/archopht.1997.01100160056009
Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995 Sep;102(9):1351-61.https://doi.org/10.1016/S0161-6420(95)30864-0
Singh AD, Eagle RC Jr, Shields CL, Shields JA. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003 Mar;121(3):397-400. https://doi.org/10.1001/archopht.121.3.397
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Drumi D. A., Poliakova S. I., Maletskiy А.P., Chebotariov Ie.P., Artemov О.V.

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) that allows users to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author as long as they cite the source.
COPYRIGHT NOTICE
Authors who publish in this journal agree to the following terms:
- Authors hold copyright immediately after publication of their works and retain publishing rights without any restrictions.
- The copyright commencement date complies the publication date of the issue, where the article is included in.
DEPOSIT POLICY
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) during the editorial process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work with an acknowledgement of its initial publication in this journal.
- Post-print (post-refereeing manuscript version) and publisher's PDF-version self-archiving is allowed.
- Archiving the pre-print (pre-refereeing manuscript version) not allowed.