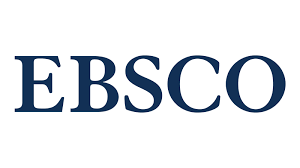Impact of a course of injections with melatonin on morphological and functional changes in the optic nerve in experimental animals with hypopinealism
DOI:
https://doi.org/10.31288/oftalmolzh202344854Keywords:
optic nerve atrophy, around-the-clock light, hypopinealism, melatonin, morphological and functional changesAbstract
Background: Optic atrophy (OA) may be expected in hypopinealism, which is accompanied by degenerative changes in the retina.
Purpose: To assess the impact of a course of injections with melatonin on the morphological and functional optic nerve (ON) changes in rabbits exposed to prolonged around-the-clock light (ATCL) leading to hypopinealism.
Material and Methods: Eighty-four rabbits were used in this experimental study. Group 1 (an ATCL group) was composed of 32 animals exposed to ATCL to develop functional hypopinealism. Group 2 (an ATCL+M group) was composed of 29 animals exposed to ATCL but treated with intramuscular melatonin for 14 days. Group 3 (a control group or CG) was composed of 23 intact animals maintained under natural day/night cycle conditions. Groups were subdivided into subgroups based on experimental constructs (1-2 months, 3-5 months, 8-12 months, 18-19 months, 26-28 months). Blood melatonin levels were assessed by commercially available enzyme-linked immunosorbent assay kits. ON specimens were obtained and comprehensively assessed morphologically and morphometrically.
Results: Night-time blood melatonin level in experimental groups was almost six-fold lower than that in controls. Signs of abnormal ON circulation were observed at ≤12 months of ATCL exposure. ON demyelination was observed from months 3-5 of the experiment. Sclerotic and atrophic processes in the ON were observed at 28 months of ATCL exposure. In ATCL26-28 and ATCL+M26-28 subgroups, the mean relative vascular area in the intraorbital ON was significantly reduced compared to CG26-28 (2.01 ± 0.15% and 1.93 ± 0.15%, respectively, versus 3.20 ± 0.13%, р < 0.05). In addition, the mean relative area of the perivascular connective tissue (4.80 ± 0.15% and 4.61 ± 0.17%, respectively) was significantly increased compared to CG26-28 (3.40 ± 0.14%, р < 0.05). Moreover, the mean diameter of the nerve fiber bundle (2.51 ± 0.09 ×10-6 m and 2.73±0.10×10-6 m, respectively) was significantly reduced compared to CG26-28 (3.85±0.14×10-6 m; р < 0.05).
Conclusion: The morphological findings (like demyelination of nerve fibers and thinning of nerve fiber bundles of the ON), combined with low blood flow in ON vessels, vascular wall thickening and connective tissue growth, indicated the development of sclerotic atrophy of the ON, in the presence of marked melatonin deficiency, in rabbits exposed to ATCL. The 14-day course melatonin treatment of ATCI-exposed rabbits exerted anti-edematous effects at early time points (< 5 months), until obviously irreversible changes in the ON occurred. However, the course melatonin treatment exerted no impact on the development of OA in animals with persistent, marked hypopinealism developed in the presence of prolonged (28-month) exposure to ATCI.
References
Quigley HA, Anderson DR. The histologic basis of optic disk pallor in experimental optic atrophy. Am J Ophthalmol. 1977;83(5):709-17. https://doi.org/10.1016/0002-9394(77)90138-6
Zhaboiedov GD, Skripnik RL. [Lesions of the optic nerve]. Kyiv: Zdorov'ie; 2006. Ukrainian.
Li HJ, Sun ZL, Yang XT, Zhu L, Feng DF. Exploring Optic Nerve Axon Regeneration. Curr Neuropharmacol. 2017; 15(6):861-73. https://doi.org/10.2174/1570159X14666161227150250
Nedzvetskaia OV, Kolot AV, Bondarenko LA. [Study on the morphological characteristics of the retina in experimental hypopinealism]. Oftalmologiia. Vostochnaia Ievropa. 2015; 2(25):35-40. Russian.
Wetterberg L. Light and biological rhythms in man. J Intern Med. 1994; 235(1):5-19. https://doi.org/10.1111/j.1365-2796.1994.tb01027.x
Korf HW, Schomerus C, Stehle JH. The pineal organ, its hormone melatonin, and the photoneuroendocrine system. Adv Anat Embryol Cell Biol. 1998; 146:1-100. https://doi.org/10.1007/978-3-642-58932-4_1
Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001; 21:6405-12. https://doi.org/10.1523/JNEUROSCI.21-16-06405.2001
Gooley JJ, Rajaratnam SM, Brainard GC. et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010; 2:31-3. https://doi.org/10.1126/scitranslmed.3000741
Reiter RJ. The pineal gland and melatonin in relation to aging: A summary of the theories and the data. Exp Gerontol. 1995; 30:199-212. https://doi.org/10.1016/0531-5565(94)00045-5
Wiechmann AF, Bok D, Horwitz J. Melatonin-binding in the frog retina: autoradiographic and biochemical analysis. Invest Ophthalmol. 1986; 27:153-63.
Cahill GM, Besharse JC. Light-sensitive melatonin synthesis by Xenopus photoreceptors after destruction of the inner retina. Vis Neurosci. 1992; 8:487-90. https://doi.org/10.1017/S0952523800005009
Martin XD, Malina HZ, Brennan MC. et al. The ciliary body - the third organ found to synthesize indoleamines in humans. Eur J Ophthalmol. 1992; 2:67-72. https://doi.org/10.1177/112067219200200203
Hamm HE, Menaker M. Retinal rhythms in chicks: circadian variation in melatonin and serotonin N-acetyltransferase activity. Proc Natl Acad Sci USA. 1980; 77:4998-5002. https://doi.org/10.1073/pnas.77.8.4998
Ruby NF, Brennan TJ, Xie X et al. Role of melanopsin in circadian responses to light. Science. 2002; 298:2211-13. https://doi.org/10.1126/science.1076701
Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005; 9:11-24. https://doi.org/10.1016/j.smrv.2004.08.001
White MP, Fisher LJ. Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vision Res. 1989; 29:167-79. https://doi.org/10.1016/0042-6989(89)90122-3
Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013; 114:25-34. https://doi.org/10.1016/j.exer.2012.12.013
Pescosolido N, Gatto V, Stefanucci A. et al. Oral treatment with the melatonin agonist agomelatine lowers the intraocular pressure of glaucoma patients. Ophthalmic Physiol Opt. 2015; 35:201-5. https://doi.org/10.1111/opo.12189
Crooke A, Guzman-Aranguez A, Mediero A, Alarma-Estrany P, Carracedo G, Pelaes T, et al. Effect of melatonin and analogues on corneal wound healing: involvement of Mt2 melatonin receptor. Curr Eye Res. 2015; 40:56-65. https://doi.org/10.3109/02713683.2014.914540
Bai J, Dong L, Song Z. The role of melatonin as an antioxidant in human lens epithelial cells. Free Radic Res. 2013; 47:635-42. https://doi.org/10.3109/10715762.2013.808743
Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: location, second messengers and role in ocular physiology. Pharmacol Ther. 2007; 113:507-22. https://doi.org/10.1016/j.pharmthera.2006.11.003
Mishchenko TV, Gladkykh OI, Poltorak VV, Bondarenko LO. [Hypopinealism as a factor of metabolic syndrome]. Endocrynologiia. 2015; 20(2):494-500. Ukrainian.
Bondarenko LA, Gubina-Vakulik, Sotnik NN. [Impact of around-the-clock illumination on circadian rhythm of melatonin and pineal gland structure in rabbits]. Problemy endocrinnoi patologii. 2005; 4:38-45. Ukrainian.
Eren TC, Reiter RJ. Light hygiene: time to make preventive use of insights old and new into the nexus of the drug light, melatonin, clocks, chronodisruption and public health. Med Hypotheses. 2009; 73:537-41. https://doi.org/10.1016/j.mehy.2009.06.003
Bondarenko LA, Sotnyk NM. [Changes in daily hormonal rhythms of the thyroid gland induced by prolonged around-the-clock light exposure]. Problemy endocrinnoi patologii. 2010; 4(34):71-7. Ukrainian. https://doi.org/10.21856/j-PEP.2010.4.10
Nedzvetska OV, Pastukh UA, Kihtenko EV, Pastukh IV, Sotnik NN, Goncharova NA, Kuzmina de Gutarra OV. Morphological and functional changes in the rabbit iris and ciliary body in experimental hypopinealism. J Ophthalmol (Ukraine). 2022; 2(505):42-7. Ukrainian. https://doi.org/10.31288/oftalmolzh202224247
Gubina-Vakulik GI, Bondarenko LA, Sotnik NN. [Prolonged around-the-clock illumination as a factor of accelerated aging of the pineal gland]. Uspekhi gerontologii. 2007; 20(1): 92-5. Russian.
Matviienko AV, Stepanova LV. [Guidelines on preclinical morphological studies in preclinical medication trials]. Kyiv: State Pharmacological Center at the Ministry of Health of Ukraine; 2001. Ukrainian.
Lillie RD. [Histopathologic technic and practical histochemistry]. Moscow: Mir; 1969. Russian.
Sarkisov DS, Perova IuL, editors. [Microscopic technique: a manual]. Moscow: Meditsina; 1996. Russian.
Lapach SN, Chubenko AV, Babich PN. [Statistical methods in medical and biological studies using Excel]. 2nd ed. Kyiv: Morion; 2007. Russian.
Avtandilov GG. [Basics of quantitative pathological anatomy]. Moscow: Meditsina; 2002. Russian.
Sergiienko VI., Bondareva IB. [Mathematical statistics in clinical studies]. Moscow: Geotar Meditsina; 2000. Russian.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Nedzvetska O. V., Pastukh U. A., Kuzmina de Gutarra O. V., Pastukh I. V., Soboleva I. A., Sotnyk N. M.

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) that allows users to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author as long as they cite the source.
COPYRIGHT NOTICE
Authors who publish in this journal agree to the following terms:
- Authors hold copyright immediately after publication of their works and retain publishing rights without any restrictions.
- The copyright commencement date complies the publication date of the issue, where the article is included in.
DEPOSIT POLICY
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) during the editorial process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work with an acknowledgement of its initial publication in this journal.
- Post-print (post-refereeing manuscript version) and publisher's PDF-version self-archiving is allowed.
- Archiving the pre-print (pre-refereeing manuscript version) not allowed.