Toxoplasma chorioretinitis in primary myeloperoxidase MPO deficiency: A case report
DOI:
https://doi.org/10.31288/oftalmolzh201947581Keywords:
chorioretinitis, toxoplasmosis, myeloperoxidase deficiencyAbstract
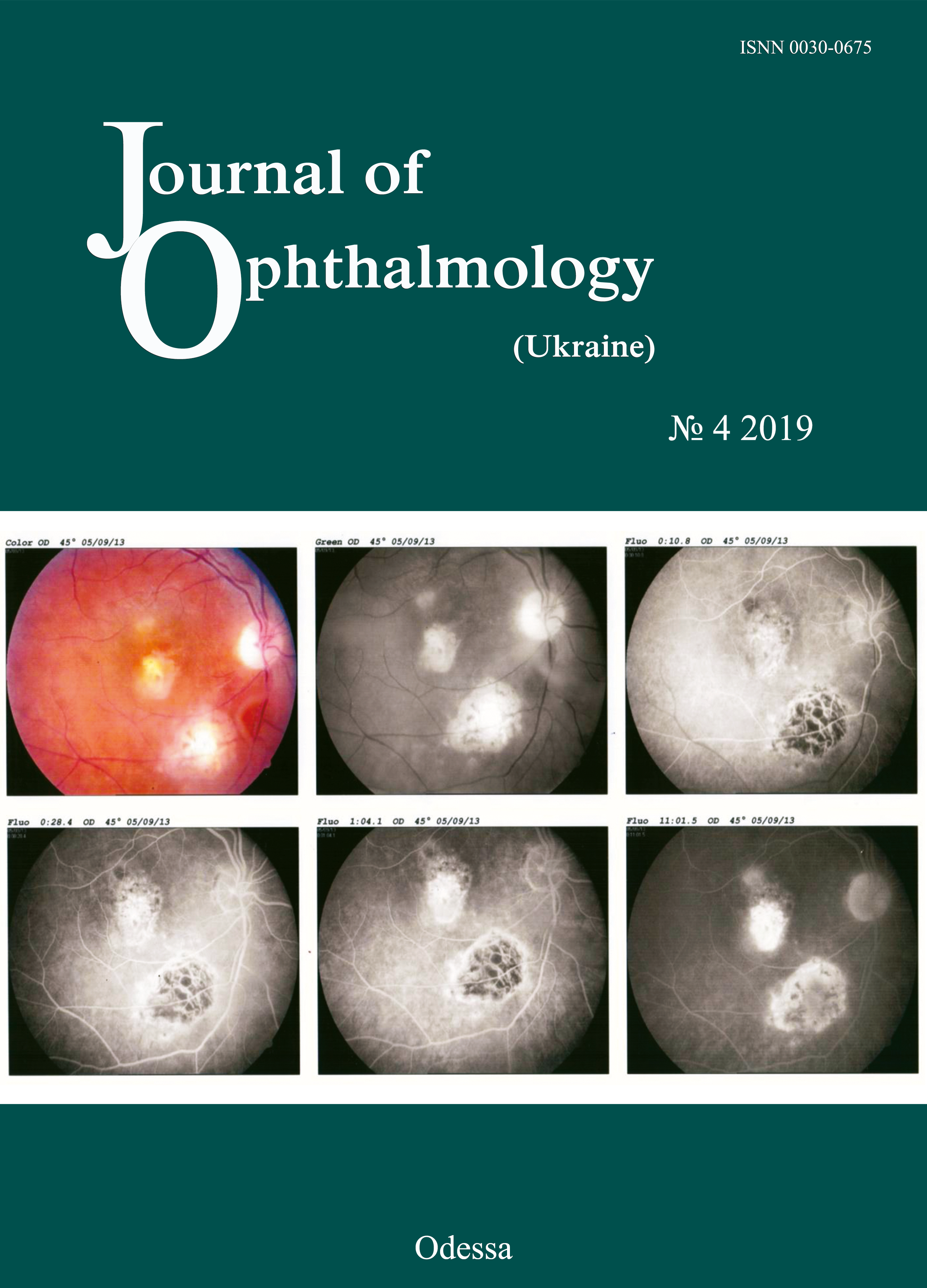
We present a clinical case of primary partial myeloperoxidase (MPO) deficiency in a young female patient with recurrent episodes of opportunistic infections. Initially, she presented to the clinical immunologist (DVM) seeking help with her severe clinical manifestations of chronic fatigue syndrome. Blood polymerase chain reaction (PCR) was positive for Epstein Barr virus. Brain MRI showed signs of external hydrocephalus and bilateral hippocampal sclerosis. After double antiviral therapy with valacyclovir (3 g daily, orally) and recombinant human alpha-2b-interferon (3 mln units every other day intramuscularly) for 3 months, the blood PCR became negative, and clinical symptoms completely resolved. A year later, the patient presented again to the immunologist, but this time due to abruptly decreased vision in her right eye. She was referred to the ophthalmologist. There were ophthalmoscopic and fluorescein angiography (FA) signs of acute chorioretinitis in the right eye. Serological testing revealed increased IgM titers to Toxoplasma gondii. Blood PCR was positive for Toxoplasma gondii. The patient was diagnosed with acute Toxoplasma chorioretinitis based on the clinical-and-laboratory, ophthalmoscopic and FA findings, and was treated for toxoplasmosis with spiramycin (1.5 mln units diluted in 200 ml of 0.9% normal saline, daily for 14 days, intravenously, and thereafter, 1000 mg three times a day after meals, for 14 days, orally). In addition, she received continuous basic immunomodulating therapy with recombinant human interferon gamma (500,000 units every other day at bedtime for 6 months) for compensation of MPO deficiency, as per the national guidelines. This normalized the percentage content and functional activity of MPO in neutrophils, and enabled prevention of further opportunistic infection episodes. This case report demonstrates that treatment of eyes affected by opportunistic infections in immunocompromised patients requires cooperation between ophthalmologists and clinical immunologists.
References
1.Nauseef WM. Diagnostic assays for myeloperoxidase and myeloperoxidase deficiency. Methods Mol Biol. 2014;1124:537-46.https://doi.org/10.1007/978-1-62703-845-4_32
2.Dinauer MC. Disorders of neutrophil function: an overview. Methods Mol Biol. 2014;1124:501-15.https://doi.org/10.1007/978-1-62703-845-4_30
3.Xiao X, Saha P, Yeoh BS, et al. Myeloperoxidase deficiency attenuates systemic and dietary iron-induced adverse effects. J Nutr Biochem. 2018 Dec;62:28-34.https://doi.org/10.1016/j.jnutbio.2018.08.003
4.Pahwa R, Jialal I. Myeloperoxidase Deficiency. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019-2018 Oct 27.
5.Kazmirchuk VE, Tsaryk VV, Maltsev DV. Myeloperoxidase deficiency - congenital and acquired disorders of neutrophil function. Fundamental and applied sciences today. 2014;3:32-6.https://doi.org/10.31640/LS-2014-(1-2)-01
6.Domingues-Ferreira M, Levy A, Barros NC, et al. Case report of myeloperoxidase deficiency associated with disseminated paracoccidioidomycosis and peritoneal tuberculosis. Rev Soc Bras Med Trop. 2017 Jul-Aug;50(4):568-570.
https://doi.org/10.1590/0037-8682-0462-2016
7.Grossl NA, Candel AG, Shrit A, Schumacher HR. Myeloperoxidase deficiency and severe sepsis. South Med J. 1993 Jul;86(7):832-6.https://doi.org/10.1097/00007611-199307000-00025
8.Ohno H. Association of primary myeloperoxidase deficiency and myeloproliferative neoplasm. Intern Med. 2010;49(22):2527-8.https://doi.org/10.2169/internalmedicine.49.3989
9.Kalinski T, Jentsch-Ullrich K, Fill S, et al. Lethal candida sepsis associated with myeloperoxidase deficiency and pre-eclampsia. APMIS. 2007 Jul;115(7):875-80.https://doi.org/10.1111/j.1600-0463.2007.apm_600.x
10.Milligan KL, Mann D, Rump A, et al. Complete Myeloperoxidase Deficiency: Beware the "False-Positive" Dihydrorhodamine Oxidation. J Pediatr. 2016 Sep;176:204-6.https://doi.org/10.1016/j.jpeds.2016.05.047
11.El Messaoudi K, Verheyden AM, Thiry L, et al. Human recombinant myeloperoxidase antiviral activity on cytomegalovirus. J Med Virol. 2002 Feb;66(2):218-23.https://doi.org/10.1002/jmv.2132
12.Chochola J, Yamaguchi Y, Moguilevsky N, et al. Virucidal effect of myeloperoxidase on human immunodeficiency virus type 1-infected T cells. Antimicrob Agents Chemother. 1994 May;38(5):969-72.https://doi.org/10.1128/AAC.38.5.969
13.Pat?ro?lu T, G?ng?r HE, Belohradsky JS, et al. Myeloperoxidase deficiency: the secret under the flag of unstained cell. Turk J Haematol. 2013 Jun; 30(2): 232-3.https://doi.org/10.4274/Tjh.2012.0012
14.Bell AL, Markey GM, Alexander HD, et al. Myeloperoxidase deficiency in a patient with rheumatoid arthritis: oxygenation and radical activity by phagocytic cells. Br J Rheumatol. Br J Rheumatol. 1993 Feb;32(2):162-5.https://doi.org/10.1093/rheumatology/32.2.162
15.Rudolph TK, Wipper S, Reiter B. et al. Myeloperoxidase deficiency preserves vasomotor function in humans. Eur Heart J. 2012 Jul;33(13):1625-34.https://doi.org/10.1093/eurheartj/ehr193
16.Kazimirchuk VE, Maltsev DV, Slobodin TN, Golovchenko IuI. [Parkinson's syndrome in young women with myeloperoxidase deficiency of phagocytes]. Mezhdunarodnyi Nevrologicheskii Zhurnal. 2011; 1(39):15-24. Russian.
17.d'Onofrio G, Mancini R, Vallone R, et al. Acquired neutrophil myeloperoxidase deficiency: an indicator of subclinical activation of blood coagulation? Blood Cells. 1983;9(3):455-66.
18.Russo AJ, Krigsman A, Jepson B, Wakefield A. Low serum myeloperoxidase in autistic children with gastrointestinal disease. Clin Exp Gastroenterol. 2009;2:85-94.https://doi.org/10.2147/CEG.S6051
19.Kettle AJ, Winterbourn CC. Superoxide-dependent hydroxylation by myeloperoxidase. J Biol Chem. 1994 Jun 24;269(25):17146-51.https://doi.org/10.1016/S0021-9258(17)32533-4
20.Albrett AM, Ashby LV, Dickerhof N, et al. Heterogeneity of hypochlorous acid production in individual neutrophil phagosomes revealed by a rhodamine-based probe. J Biol Chem. 2018 Oct 5;293(40):15715-15724.https://doi.org/10.1074/jbc.RA118.004789
21.Azarova LA, Vasilenko LP, Oblamskaia GV. [Hereditary myeloperoxidase deficiency: its diagnosis on the Technicon H.1 hematological analyzer]. Gematol. Transfuziol.1993 Feb;38(2):41-2. Russian.
22.Becker R, Pfl?ger KH. Myeloperoxidase deficiency: an epidemiological study and flow-cytometric detection of other granular enzymes in myeloperoxidase-deficient subjects. Ann Hematol. 1994 Oct;69(4):199-203.https://doi.org/10.1007/BF02215954
23.Ro?niak-B?k K, Jele? A, B?k M. Analysis of Separation of White Blood Cells in Peripheral Blood as an Indicator of MPO Deficiency. Ann Clin Lab Sci. 2017 Aug;47(4):422-431.
24.Marchetti C, Patriarca P, Solero GP, et al. Genetic studies on myeloperoxidase deficiency in Italy. Jpn J Infect Dis. 2004 Oct;57(5):S10-2.https://doi.org/10.7883/yoken.JJID.2004.S10
25.[Locally Adapted National Evidence-based Guidelines for Managing Myeloperoxidase Deficiency. State Expert Center of the Ministry of Health of Ukraine and Ukrainian Society of Specialists for Immunology, Allergology and Immunorehabilitation]. Ukrainian. Available at http://mtd.dec.gov.ua/images/dodatki/2016_609_DefMiel_Fago/2016_609_AKN_.
26.[Unified Clinical Pathway for Providing Care to Patients with Myeloperoxidase Deficiency]. 2016. Ukrainian. Available at http://mtd.dec.gov.ua/images/dodatki/2016_609_DefMiel_Fago/2016_609_YKPM.
27.Maltsev DV. [Efficacy of long-term continuous immunomodulating therapy with recombinant human interferon gamma for patients with clinically manifested forms of myeloperoxidase deficiency]. Immunologiia I allergologiia. 2015; 1:44-53. Russian.
28.Maltsev DV. 2015. The effectiveness of combined antiviral therapy in chronic mononucleosis caused by Epstein-Barr virus. European EBV meeting. Karolinska Institutet. Stockholm. p. 33.
29.Ramsaransing G, Teelken A, Prokopenko VM. Low leucocyte myeloperoxidase activity in patients with multiple sclerosis. J Neurol. Neurosurg Psychiatry. 2003;74(7):953-5.https://doi.org/10.1136/jnnp.74.7.953
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Д. В. Мальцев, О. О. Гуржій

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) that allows users to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author as long as they cite the source.
COPYRIGHT NOTICE
Authors who publish in this journal agree to the following terms:
- Authors hold copyright immediately after publication of their works and retain publishing rights without any restrictions.
- The copyright commencement date complies the publication date of the issue, where the article is included in.
DEPOSIT POLICY
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) during the editorial process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work with an acknowledgement of its initial publication in this journal.
- Post-print (post-refereeing manuscript version) and publisher's PDF-version self-archiving is allowed.
- Archiving the pre-print (pre-refereeing manuscript version) not allowed.